(i) ‘B’ is preferred for cleansing action. The calcium and magnesium salts present in underground water are precipitated with carboxylic acids. The Ca++ and Mg++ salts of sulphonic acid are soluble in water. ‘B’ is a more effective cleansing agent in presence of Ca and Mg salts.
(ii) Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons.
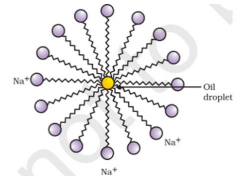
The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap dissolves in water while the carbon chain dissolves in oil. The soap molecules, thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water and we can wash our clothes clean.
(iii) CH3COOCH3+ NaOH -----> CH3COONa+ CH3OH
The process is saponification.