(i) Keerthi’s thinking is correct as substitution reactions occur in saturated hydrocarbons, hydrogen atoms are replaced with heteroatoms in saturated hydrocarbons. Whereas in unsaturated hydrocarbons an addition reaction occurs, simple molecules are added across double and/or triple bonds.
(ii) Methane and propane undergo combustion reaction in presence of oxygen and produce large amount of energy.
The lower homologue of propane is ethane has the following electron dot structure:
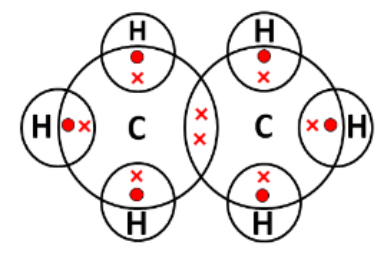
Any two characteristics -
- Difference in -CH2- / 14u molecular mass of any two adjacent homologues.
- Same general formula/ functional group -
- Similar chemical properties -
- Gradual change in physical properties
(iii) The mixture of ethyne and oxygen in sufficient amounts undergoes complete combustion to fire a clean blue flame. In pressure of insufficient supply of oxygen or in the presence of air, ethyne does not undergo complete combustion and produces sooty flame.